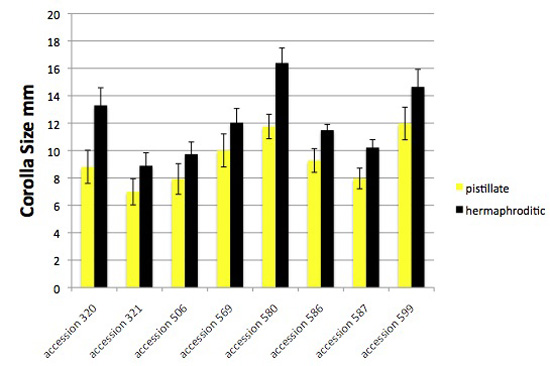
Figure 1. Corolla size varies among accessions of Jaltomata procumbens, and is smaller during the pistillate phase, becoming significantly* larger the next day when flowers become functionally hermaphroditic. The plants from which this data was obtained also served as parents in manual hybridizations (year 2012) for genetics.
"pistillate" - corolla radius was measured during the first day a flower was open: filaments were shortest and all anthers remained undehisced
"hermaphroditic" - corolla radius was measured after filaments elongated and all anthers had dehisced, the second phase of the flower's life.
Why does the corolla expand while making the transition from the pistillate phase to the hermaphroditic phase, a day after it opens? Here I speculate:
A pistillate-phase flower needs only one or a few visits to be pollinated and set fruit, while a flower providing pollen can contribute to a plant’s fitness during multiple visits by pollinators. Given that a pistillate phase is followed by a hermaphroditic phase, a plant can achieve greater fitness by doing something to attract more visits to pollen-providing flowers than to pistillate-phase flowers. Having pistillate flowers the same size as hermaphroditic flowers would invite an equal number of visits per unit time (both phases provide nectar). And these limited number of visits would be better spent asymmetrically, by directing pollinators preferentially to flowers that provide pollen because a flower providing pollen can contribute to a plant’s fitness the most by receiving multiple visits by pollinators. Given a finite number of insect visits, more than a few insect visits to a flower that is pistillate has the cost of lost visits to flowers where pollen could have been picked up and carried elsewhere resulting in the siring of seeds.
Vertical bars extend one standard deviation above and below the mean.
*T-tests, 1-tailed, were significant, p < 0.0001 for all tests, one test per accession. A Wilcoxon matched-pairs signed-ranks test, pistillate mean corolla lengths
vs the hermaphroditic mean corolla lengths, each pair of values coming from one accession, one-tailed, was also significant with p = 0.0039 (W = -36, T+ = 0.0, T- = -36, 8 pairs; nonparametric Spearman r = 0.9048 shows effective pairing). And, an unpaired t test that does not assume equal variances gave a p value of 0.0145, t = 2.479, 12 df, Welch correction applied).
Measurements made on outdoor and indoor plants were pooled, and were from the base of the androecium to the corolla lobe's tip while the ruler was flat to the corolla. Each column is based on the following number of measurements: accession 320 (18,20), accession 321 (16, 24), accession 506 (13,13), accession 569 (12,13), accession 580 (11,11), accession 586 (8,9), accession 587 (13,16), accession 599 (22,23). All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
Figure 2. Calyx size varies among accessions of Jaltomata procumbens, and remains the same* size while a flower is open. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
"pistillate" - calyx was measured during the first day a flower was open: filaments were shortest and all anthers remained undehisced
"hermaphroditic" - calyx was measured after filaments elongated and all anthers had dehisced, the second phase of the flower's life.
Vertical bars extend one standard deviation above and below the mean. Measurements made on outdoor and indoor plants were pooled, and were from the base of the pedicel to the calyx lobe's tip while the ruler was flat to the calyx.
*T-tests, 2-tailed, were all not significant, p > 0.06 for all T-tests, one T-test per accession.
Each column is based on the following number of measurements: accession 320 (17, 17), accession 321 (16, 22), 506 (12, 13), 569 (9, 11), 580 (11, 9), accession 586 (8, 10), accession 587 (12, 12), accession 599 (23, 20). All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
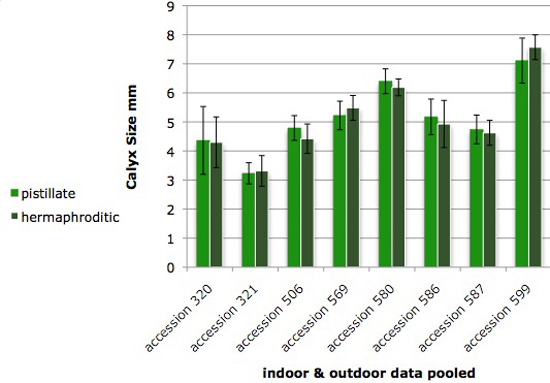
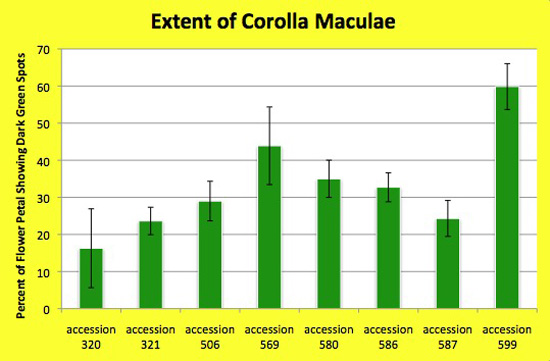
Figure 3. The extent of darker green spots (pigmentation) on the lighter green corolla varies among accessions of Jaltomata procumbens, as shown in both this graph and figure 11 below. Measurements were made while a ruler was flat to the corolla, using only flowers in the hermaphroditic phase (all anthers had dehisced). Data from outdoor and indoor plants were pooled. Each value is from one flower and is: (the length of the darker green spots from the androecium toward the tip of the petal / the length of the petal) X 100.
A Kruskal-Wallis Test (nonparamentric Anova) gave a p value < 0.0001 indicating that variation among accessions (columns) is significantly greater than expected by chance.
The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
To consider the constancy of this character I compared the extent of corolla spots on a flower of an herbarium specimen with the data gathered for this graph. Good news! The measurement on the herbarium specimen I made in 1994 was very nearly the mean value of measurements made in 2012 (accession 580)!
Each column is based on the following number of maculae length / petal length measurements: accession 320 (18), accession 321 (22), accession 506 (11), accession 569 (10), accession 580 (11), accession 586 (9), accession 587 (13), accession 599 (23). Vertical bars extend one standard deviation above and below the mean. The data for accession 320 is highly variable because maculae did not form on flowers of plant B grown in the UConn greenhouse. All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
"pistillate" - filaments were measured during the first day a flower was open: filaments were shortest and all anthers remained undehisced
"hermaphroditic" - filaments were measured after filaments elongated and all anthers had dehisced, the second phase of the flower's life.
Vertical bars extend one standard deviation above and below the mean. Measurements made on outdoor and indoor plants were pooled, and were from the base of the androecium to the distal end of the filament. "Filament length" includes the expanded base of the stamen; in other words the zero of the ruler was touching the corolla when then filament length was measured.
Each column is based on the following number of measurements: accession 320 (13, 14), accession 321 (13, 18), accession 506 (8, 7), accession 569 (6, 8), accession 580 (7, 10), accession 586 (6, 4), accession 587 (7, 3), accession 599 (19, 10).
All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
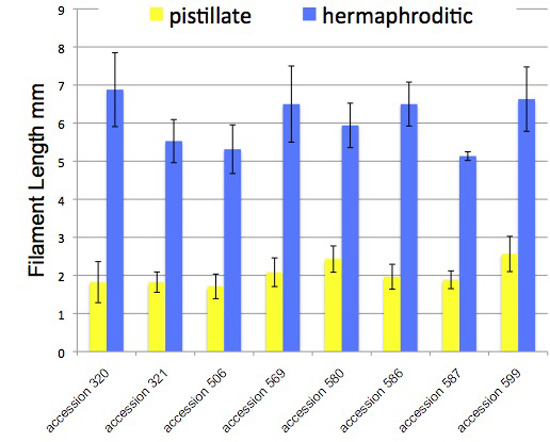
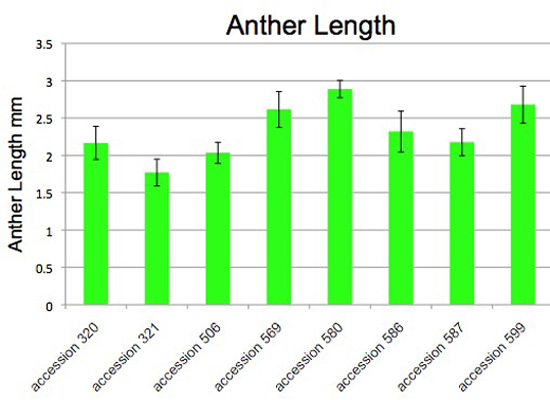
Figure 5. Anther length varies among accessions of Jaltomata procumbens. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
Vertical bars extend one standard deviation above and below the mean. Both one-way Analysis of Variance (ANOVA) and a Kruskal-Wallis Test (Nonparamentric Anova) gave p values < 0.0001 indicating that variation among accessions (columns) is significantly greater than expected by chance.
Prior to dehiscence fresh (not preserved, not pressed) anthers were measured with a dissecting microscope. Measurements made on outdoor and indoor plants were pooled. The same microscope, fixed at one magnification, was used for all measurements.
Each column is based on the following number of measurements: accession 320 (19), accession 321 (17), accession 506 (15), accession 569 (12), accession 580 (10), accession 586 (10), accession 587 (13), accession 599 (26). All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
Figure 6. Percent of filament's length having trichomes, proximal to distal, varies among accessions of Jaltomata procumbens. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
Flowers were in the hermaphroditic phase (filaments had elongated, all anthers had dehisced). One flower was used for each value. Each value was: (the length of the part of the filament having trichomes divided by the length of the filament including its expanded base) X 100. Measurements made on outdoor and indoor plants were pooled.
Vertical bars extend one standard deviation above and below the mean.
Each column is based on the following number of measurements: accession 320 (14), accession 321 (18), accession 506 (7), accession 569 (8), accession 580 (10), accession 586 (4), accession 587 (3), accession 599 (10).
All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
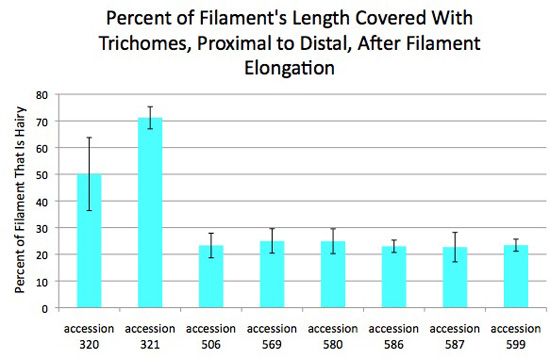
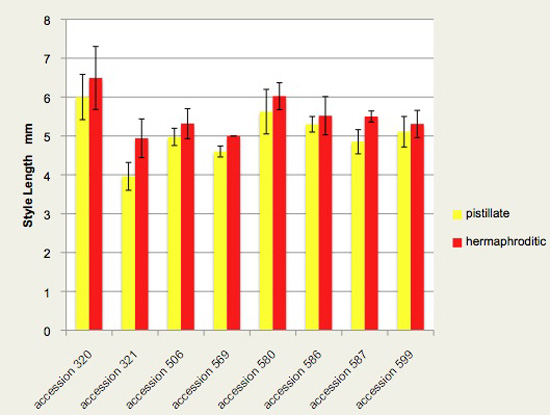
Figure 7. Style length varies among accessions of Jaltomata procumbens, and significantly* increases in length during the transition from a flower's pistillate phase to its hermaphroditic phase. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
"pistillate" - style was measured during the first day a flower was open: filaments were shortest and all anthers remained undehisced
"hermaphroditic" - style was measured after filaments elongated and all anthers had dehisced, the second phase of the flower's life.
*A paired t test was significant, pistillate mean style lengths vs hermaphroditic mean style lengths, each pair of values coming from one accession (p = 0.0007, t = 5.108, 7 df; pairing was effective with r = 0.9173). And, a Wilcoxon matched-pairs signed-ranks test, each pair of values coming from one accession, one-tailed, was also significant with p = 0.0039 (W = -36, T+ = 0.0, T- = -36, 8 pairs; nonparametric Spearman r = 0.9048 shows effective pairing).
I know of no functional explanation as to why the style would, after the flower opens, grow slightly. Quite possibly, pleiotropy: there are genes that control the timing of stamen elongation (a day after-the-flower-opens), and stamen elongation has a functional explanation (Figure 4). Perhaps style elongation happens just because the same gene(s) are acting on both.
From this ministudy, we now know that for genetics styles should be measured during one phase or the other consistently, not both phases.
Vertical bars extend one standard deviation above and below the mean. Styles were measured by removing a flower and then carefully removing the style from the ovary. The style was then placed on a ruler and measured to the nearest tenth of a mm while looking through either a hand lens or a dissecting microscope. Each column is based on the following number of measurements: accession 320 (11,12), accession 321 (5,5), accession 506 (4,6), accession 569 (1,2), accession 580 (8,9), accession 586 (3,5), accession 587 (2,4), accession 599 (12,13). All measurements by Thomas Mione in the University of Connecticut greenhouse 2012.
Figure 8. Stigma diameter varies among accessions of Jaltomata procumbens. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
Fresh (not preserved, not pressed) stigmas were measured with a dissecting microscope while the style was resting on the microscope's stage plate, thus the view while measuring was perpendicular to the style's length. Measurements made on outdoor and indoor plants were pooled.
Vertical bars extend one standard deviation above and below the mean. The same microscope, fixed at one magnification, was used for all measurements.
Each column is based on the following number of measurements: accession 320 (24), accession 321 (31), accession 506 (18), accession 569 (15), accession 580 (12), accession 586 (11), accession 587 (13), accession 599 (31).
All measurements by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor planting boxes in New Britain, CT.
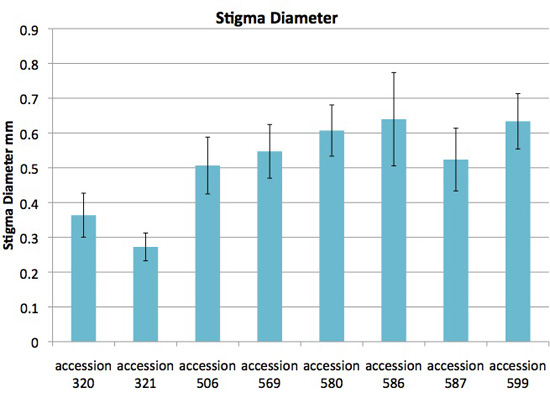
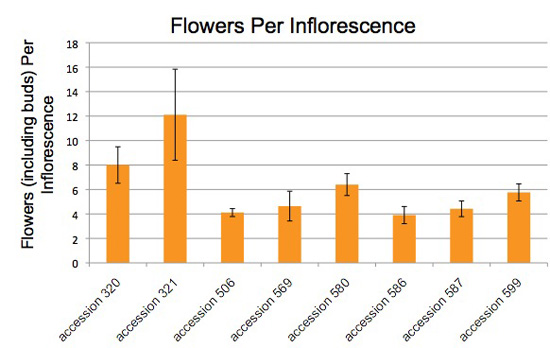
Figure 9. The number of flowers per inflorescence varies among accessions of Jaltomata procumbens. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
The number of flowers per inflorescence was counted using fresh (not preserved, not pressed) inflorescences. Counts made on outdoor and indoor plants were pooled, and data from plants grown in previous years were included.
Vertical bars extend one standard deviation above and below the mean.
Each column is based on the following number of measurements: accession 320 (10), accession 321 (9), accession 506 (18), accession 569 (14), accession 580 (5), accession 586 (11), accession 587 (26), accession 599 (21).
All counts by Thomas Mione in the University of Connecticut greenhouse, the outdoor garden of the University of Connecticut, and outdoor gardens in central CT.
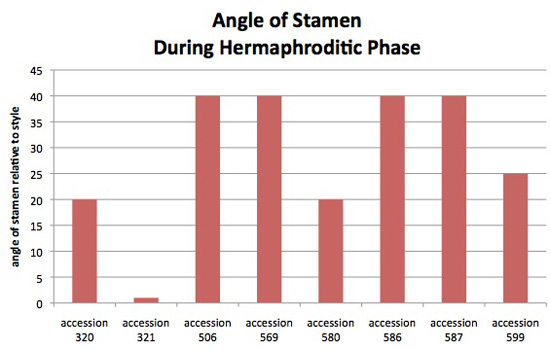
Figure 10. Stamen angle relative to the style. This assessment was made during the flower's hermaphroditic phase, mid-day because with the normal closure of the corolla at dusk the angle decreases.
Flowers were scored as having stamens that angle out strongly, angle out somewhat, or connivent stamens. An example of stamens that angle out strongly can be seen on the right side of figure 1 of the J. procumbens page. Examples of filaments that angle out somewhat can be seen in figures 6 & 15 of the J. procumbens page. Connivent stamens can be seen on the Jaltomata 321 page. These three categories, strongly, somewhat and connivent, were converted to approximate values to make this graph. The plants from which this data was obtained also served as parents in manual hybridizations for genetics.
Each column is based on observations made on the following number of plants/flowers: accession 320 (5/11), accession 321 (6/7, and many flowers and plants in previous years), accession 506 (7/11), accession 569 (5/8), accession 580 (2/2), accession 586 (3/5), accession 587 (5/9), accession 599 (6/13).