
Seeds collected in Ancash, Peru in 2015, Mione Leiva G. & Yacher 855 or 856, plant grown in USA, photo by T. Mione.
(photo by Thomas Mione, Mione Leiva G. & Yacher 758, plant grown in Connecticut, USA)

Jaltomata calliantha S. Leiva & Mione |
Peru |
revised Aug 2023 |
| Link to Jaltomata homepage |
The information on this page may be cited as a communication with professor Thomas Mione, |
Links to Jaltomata of Ancash and La Libertad, Peru |
Link to the Jaltomata species
of northern Peru |
Link to the Jaltomata species having red / orange nectar |
Link to the local names of Jaltomata species, including this one |
 |
|---|
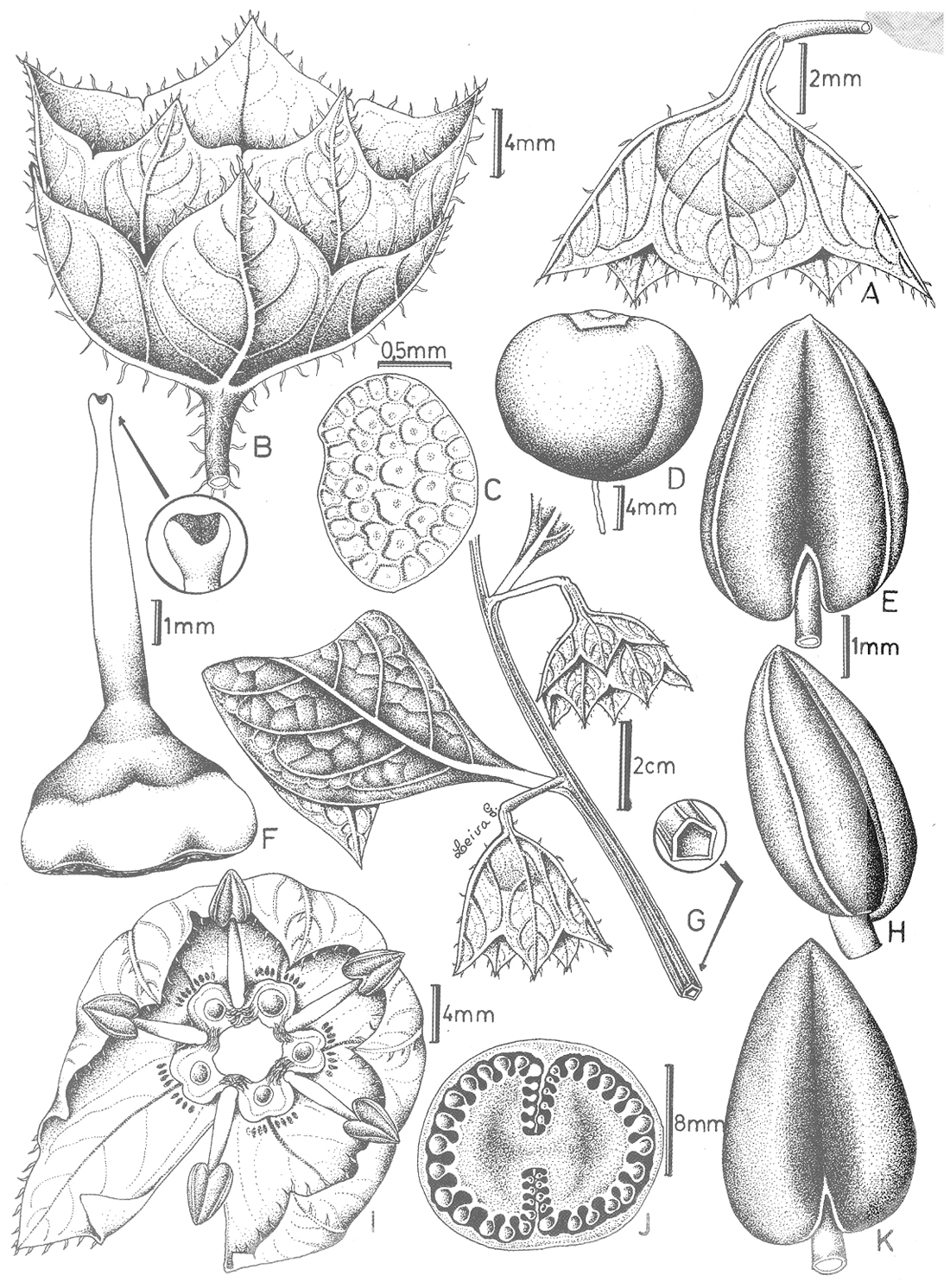
| Figure 1, above. Flower producing red nectar. Anthers all dehisced. Seeds collected in Ancash, Peru in 2015, Mione Leiva G. & Yacher 855 or 856, plant grown in USA, photo by T. Mione. |
| Figure 2, above. Red nectar pools not on the base of the corolla, but on a corona (bowl of tissue possibly formed by the fused bases of the stamens). Anthers have all dehisced. Nectar darkens with age (photo by Thomas Mione, Mione Leiva G. & Yacher 758, plant grown in Connecticut, USA) |
 |
| Figure 3. Note that anthers are undehisced in the Day 1 flower. Photo by Jamie Kostyun, Mione & Leiva 711 |
| Figure 4. The corona (bowl of tissue on which nectar pools) is clearly evident. Nectar is clear (not red/orange) when a flower first opens; anthers undehisced (photo by Thomas Mione, Mione Leiva G. & Yacher 758) | Figure 5. Nectar is clear (not red/orange) when a flower first opens; anthers undehisced (photo by Thomas Mione, Mione Leiva G. & Yacher 758) |
|---|
| Figure 6, above. Bud in upper left shows sepals fully open before corolla opens. Open flower at top right is in pistillate phase, showing clear nectar and undehisced anthers. Flower at bottom is in the hermaphroditic phase, showing orange nectar and dehisced anthers (photo by Thomas Mione, Mione Leiva G. & Yacher 758). |
|---|
| Figure 7, above. Jaltomata calliantha is shown slightly to the right of center, growing in the protection of a spiny Puya. Hummingbirds reapeatedly visted the flowers. We determined, by remaining at the site and making observations, that the only flowers that had nectar were those that were not found by the hummingbirds, others were lacking nectar because the birds were repeatedly visiting and removing nectar by visiting as many flowers as they could find (photo by Thomas Mione, Mione Leiva G. & Yacher 758). |
| Figure 8. Habitat of Jaltomata calliantha (Photo by Segundo Leiva G., March 2007) |
Figure 9. Photo taken while we were on our way to the habitat of J. calliantha, on foot, from the nearest road. Fog and clouds coming in before rain. Segundo Leiva G. at right (photo by Thomas Mione, March 2007) |
|---|
| Flower solitary, large 3-4 cm across, campanulate and green, nectar orange/red, corona on which nectar pools, mature fruit very pale-green, stigma punctiform (not capitate), the style gradually narrowing proximal to distal, hairs not gland-tipped (except those of seedling are gland-tipped). Herbaceous except at ground-level where a woody base was observed on cultivated plants. |
| Character | Description | Additional notes, or figures on this page |
Habit & Height |
Herbaceous, probably perennial, the above-ground parts completely dying during dry season | |
Branches, young |
||
older |
||
Leaves, size |
||
| shape | ||
| hairs | First foliage leaves of seedling are pubescent with unpigmented finger hairs, all gland-tipped (Mione et al. 855 & 856). | |
| petiole | ||
Inflorescence |
1 (very rarely 2, Kostyun personal communication May 2013, cultivation of Mione 711) |
1, 6 |
peduncle |
||
pedicel |
in fruit, conspicuously angular (Mione et al. 855) | |
Calyx at flowering |
||
| at fruit maturity | Infundibular with raised main veins that extend from pedicel to lobe tip, and large for this genus: 4.4 cm from lobe tip to lobe tip (nearly a diameter), 2 cm from pedicel to lobe tip (Mione et al. 855) | |
Corolla color |
green (similar to color of leaves) with a ring of darker green maculae at base | |
shape and size |
||
lobes/lobules |
5 | |
hairs
|
The corolla is adaxially densely pilosulose, the hairs conspicuously gland-tipped and 0.25 – 0.35 mm long on fresh material; and abaxially villous the hairs eglandular and to 2 mm long. | |
| no | ||
Stamen length including anther |
stamens are expanded at the base where they are surrounded by simple hairs; | |
| filaments | filaments purple, glabrous | |
| anther color | yellow prior to dehiscence | |
| anther size | ||
| anther mucronate / mucronulate | ||
| insertion of filament into anther | Basifixed (not lower ventral face) | |
| anthers of a flower open simultaneously? | ||
| pollen grain size | 22.5 - 26.25 micrometers, mean 24.68 micrometers |
Mione et al. 758, June 2012 by a student 15 grains, flowers collected in Peru |
| corona | yes | 1, 2, 3, 4, 5, 6 |
Stigma |
punctiform | |
Style |
widest at base and gradually narowing toward apex, very rigid | |
Ovary |
||
Nectar |
Orange-red | |
| Herkogamy | Yes, the dehisced anthers are held several mm away from the stigma | |
| Protogyny | yes | |
Fruit color (at maturity) |
very pale green (observed in both Department La Libertad and Department Ancash) |
|
| Fruit Size and Seeds per fruit | 15 X 20 mm, 12 X 16 mm (Mione et al. 855) 16 X 24 mm (Mione et al. 856) 11 X15.5 mm from a plant grown in Connecticut on a windowsill in CCSU's science building. |
the first of the two numbers represents the distance from the pedicel attachment to the opposite pole; figure 2 |
Seed Size |
250 to 469 seeds (n = 2 fruits) 327 (Mione et al. 855) |
I selected one of the larger fruits, wild-collected not grown for study, for a seed count. |
| Seedling Hairs | all gland-tipped, finger hairs, scroll way down, Figure __ on this page |
Mione et al. 855 or 856, seed germinated by Rebecca Flinn |
| Character | Description of Jaltomata calliantha | Additional notes, or figures on this page |
| Character | Description | |
Growability in Connecticut, USA |
Easy | |
How long does it take from flower to ripe fruit? |
about 5 - 6 weeks |
Kostyun, personal communication 2013, cultivation of Mione 711 |
Flowers Closing For The Night? |
No | |
Self-Compatible? |
Yes, a manual self-pollination (9 Sep 2008) of a hermaphroditic-phase flower resulted in the production of one fruit containing viable-looking seeds, harvested in early November 2008. | Kostyun agrees, personal communication 2013, cultivation of Mione 711 |
| Pollen quantity per flower | a) 288,333 grains (n = 1 flower) b) 392,500 (n = 1 flower) |
a) count by Elisabeth dos Santos, 2011 |
Ovules per ovary |
489 (same flower as "b" above in pollen quantity) | Mione et al. 758, count by Thomas Mione 2012 |
Ratio of pollen to ovules |
803 | n = 1 flower: 392,500 / 489 ovules |
Chromosome number |
no data |
| Seedling Hairs | All gland-tipped finger, erect, straight, clear, estimated by unaided eye to be about 2 mm long, and covering both faces of leaves, petioles, and axes (Mione et al. 758, observations made May 2008). Hypocotyl and cotyledons of seedlings are pubescent with unpigmented conspicuously gland-tipped finger hairs |
| Ecology | In Department La Libertad it grows almost exclusively in the the protection of the spiny leaves of Puya. In Department Ancash we twice found it along a road in full sun. |
| Seed Germination (seeds were stored in the refrigerator, except for the first days after harvest when the seeds were stored at ambient temperature) |
GA used | Seed coat partially removed | Heat Mat Used? | Number of days or months until first above-ground signs of germination |
additional notes, if any |
| Mione et al. 758, planted 7 April to 29 April 2008 | no | possibly | no data | 22 days | A second seed germinated one month after the seeds were orginally planted (April/May 2008, CCSU greenhouse). The percent of seeds germinating was low (only two germinated out of about 30). |
| Mione et al. 855, planted 6 September to 29 September 2016 | no | no | yes | 23 days | |
| Mione et al. 855, planted 9 Oct 2020, first above-ground germination noted on 8 Jan 2021 | no | no | yes and no | 3 months | seeds (in potting mix and kept moist) were on a heat mat with artificial light for about two months before being moved to the greenhouse where they were kept moist but got cold at night and received a mixure of natural and artificial light duriing the day, and remained cool during the day with 10 am temperatures of 19C (and cooler unrecorded nightime temperatures). |
Similar Species: The three species of section Modillonia are herbaceous, have solitary flowers, a 5-lobed corolla, a corona (figures 1, 2, 3, 4, 5, 6, 13 of this page), lack radial thickenings, have basifixed anthers (other species of this genus have the filament inserted into the lower ventral face of anther), a stigma no wider than top of style (stigma punctiform, not capitate), grow in Peru and produce copious red-orange nectar. A table comparing the species of Jaltomata section Modillonia can be seen on the webpage of J. quipuscoae. |
Specimens Examined: all Peru, :
| Department, province | Location |
elevation m |
habitat |
collection date |
collectors |
comments |
|
| La Libertad, Otuzco | Distrito Salpo, arriba de Platanar, ruta Platanar-Pagash | 8 00' 86" S 78 41' 47" W |
1430 |
see protologue, page 169 | 11 March 2005 |
S. Leiva G. 3154 (HAO, CORD, F) | TYPE SPECIMEN |
| La Libertad, Otuzco | 4 km S of Puente Casmiche, rd from Trujillo to Otuzco | 7.8 W 78.5 S |
1870 |
sandy and rocky soil | 18 March 1999 |
D M. Spooner et al. 7315a (US, WIS) | |
| La Libertad, Otuzco | above the town of Platanar on way to town Cucualle | 1420 - 1453 |
24 Mar 2005 |
S. Leiva G. 3155 | |||
| La Libertad, Otuzco | 1453 |
S. Leiva G. 3275 | |||||
| La Libertad, Otuzco | 1425 - 1445 |
sandy, rocky soil | 9 June 2005 |
Mione, Leiva & Yacher 711 | photos only, no plant specimen; too dry in June |
||
| La Libertad, Otuzco | arriba de Platanar (Platanar-Salpo) | 8 00.790 S, 78 41.462 W |
1480 |
rocky, only found with Puya | 23 March 2007 |
Leiva & Mione 3658 Mione & Levia 758 |
DNA sample taken |
| Ancash, Huaylas | road from Moro to Pamparomas. link to movie showing habitat |
09 05.369 S, 078 04.331 W |
1,379 - 1,390 |
roadside, full sun | 17 May 2015 | T. Mione, S. Leiva G. & L. Yacher 855* S. Leiva G., T. Mione, & L. Yacher 5875 |
Herbarium specimen Mione et al. 855 was lost in transit from South to North America. There were no flowers when we collected these specimens, but photos, seeds and dry leaves for nucleic acid extraction were all taken. |
| Ancash, Huaylas | road from Moro to Pamparomas | 09 05.297 S 078 00.935 W |
2,100 |
roadside, full sun | 17 May 2015 | T. Mione, S. Leiva G. & L. Yacher 856* S. Leiva G., T. Mione, & L. Yacher 5881 |
Herbarium specimen Mione et al. 856 was lost in transit from South to North America. There were no flowers when we collected these specimens, but photos, seeds and dry leaves for nucleic acid extraction were all taken. |
| Ancash, Huaylas | Distrito Pamparomas, road from Moro to Pamparomas, km 25 | no data |
only in moderately moist, cultivated area | 4 May 2000 | M. Weigend 2000/605 (M) |
 |
| Figure 10. Jaltomata calliantha. Illustration that appears in the protologue, by Segundo Leiva G. |
| Figure 11, above. The nectar trough of Jaltomata calliantha is formed by the corona (C). Note that the nectar (N) is amber in color (true of first-day flowers). Part of the corolla and one of the stamens was removed, and three stamens were excised near the base of the filament. Seeds collected in Ancash, Peru in 2015, the plant shown in this photograph was grown for study in Connecticut. Photo by T. Mione, Mione et al. 855 or 856, fall 2015. |
 |
| Figure 12, above. Jaltomata calliantha. The corolla was pulled away during dissection, and a stamen and the corona came with it because they are adnate. Seeds collected in Ancash, Peru in 2015, the plant shown in this photograph was grown for study in Connecticut. Photo by T. Mione, Mione et al. 855 or 856, fall 2015. |
| Figure 13. View of valley from path to habitat of Jaltomata calliantha (photo by Thomas Mione, March 2007) | Figure 14. Road nearest to habitat of Jaltomata callinatha. Segundo Leiva G. outside of vehicle, Leon Yacher in driver's seat (photo by Thomas Mione) |
|---|---|
| Figure 15. The corona, the bowl of tissue on which nectar pools, is clearly evident (flower stored in ethanol, photo by Thomas Mione January 2006 in Connecticut with dissecting scope, Leiva 3154). | |
| Figure 16. Flower slightly open (photo by Thomas Mione, Mione Leiva G. & Yacher 758) |
Figure 17. Open flower in side view. Calyx is planar, corolla urceolate (photo by Segundo Leiva G.). |
 |
|
Figure 18. Above-ground parts die back during dry season. Rains come in December January and March (Segundo Leiva, personal communication). |
Figure 19. Above-ground parts were considered herbaceous when plants were encountered in Peru, but when grown in Connecticut, USA this woody stem was observed at the soil surface (photo by Thomas Mione, plant grown in USA) |
| Figure 20. Mature Fruit is green (photo by Thomas Mione, Mione Leiva G. & Yacher 758) |
|---|
 |
| Figure 21. Top leaf shows adaxial (top) face. Bottom leaf shows abaxial (bottom) face. Mione et al. 758, from seeds collected in Peru, grown on a window sill at Central Connecticut State University, scanned by Thomas Mione August 2008. |
 |
|
|---|---|
| Figure 22. Leaves (Leiva 3155, photo by Segundo Leiva G.) | Figure 23. Leaves (Leiva 3155, photo by Segundo Leiva G.) |
| Figure 24. Shoots of Jaltomata calliantha. Several flowers can be seen, albeit inconspicuous (photo by Thomas Mione, Mione Leiva G. & Yacher 758) |
|---|
| Figure 25. Jaltomata calliantha stem cross section, showing from top to bottom, collenchyma, parenchyma, secondary phloem and secondary xylem (blue). Fibers are purple, near to top of photo (plant grown in Connecticut, USA, cross section and photo by Sarah Saunders 2009, Toluidine Blue, sectioning was done by hand, stem green and 6 mm in diameter) |
| Figure 26. Secondary xylem at upper left, secondary phloem and fibers (plant grown in Connecticut, USA, cross section and photo by Sarah Saunders 2009, Toluidine Blue, sectioning was done by hand, stem 6 mm in diameter and green) |
| Figure 27. Cork (plant grown in Connecticut, USA, cross section and photo by Sarah Saunders 2009, Toluidine Blue, sectioning was done by hand, stem 12 mm in diameter and green) | Figure 28. Cross section showing, from top to bottom: epidermis, collenchyma, parenchyma, fibers (purple), secondary phloem, secondary xylem (blue) and pith at bottom (plant grown in Connecticut, USA, cross section and photo by Sarah Saunders 2009, Toluidine Blue, sectioning was done by hand, stem 6 mm in diameter and green) |
|---|
 |
|---|
| Figure 29. Flower (Mione Leiva G. & Yacher 758, plant grown in Connecticut, USA, photo by Clinton Morse at the Univ of Connecticut, 2008) |
| Figure 30. Hermaphroditic phase showing orange nectar (anthers dehsiced, photo by Thomas Mione, Mione Leiva G. & Yacher 758) |
| Figure 31. Anthers and distal portion of filament of J. calliantha collected in Department Ancash; ventral view on left, dorsal view on right; anthers undehsiced, units on ruler across top are mm, photo by Thomas Mione, Mione Leiva G. & Yacher 855 or 856. |
 |
| Figure . The corolla is adaxially densely pilosulose, the hairs conspicuously gland-tipped and 0.25 – 0.35 mm long on fresh material; and abaxially villous the hairs eglandular and to 2 mm long as shown above. Photo by T. Mione. |
| Figure 32, above. Ripe fruits, Mione et al. 855 and/or 856, units are mm, photo by T. Mione in Peru |
| Figure 33, above. Leaves, branch and ripe fruits. Mione et al. 855 and/or 856, numbered units are cm, photo by T. Mione in Peru |
| Figure 34, above. Leaves. Mione et al. 855 and/or 856, units are mm, photo by T. Mione in Peru |
| Figure 35, above. Both a peduncle and a pedicel are evident. Mione et al. 855 and/or 856, larger units are mm, photo by T. Mione in Peru |
| Figure 36, above. Mione et al. 855 and/or 856, numbered units are cm, photo by T. Mione in Peru |
| Figure 37, above. Seedling of Jaltomata calliantha from seed collected in Department Ancash. Note covering of gland-tipped finger hairs. Leaf to the upper right is 10 mm long, and lowest leaf (oriented toward viewer, out of focus) is 13 mm long. Mione 855 or 856. Seed germinated by Rebecca Flinn. Photo by T. Mione. |
Rebecca Flinn's 2015 data are shown twice, once here in text, and again in the table below. GA 150 ppm, with scarification: germination in average of 20.5 days There was no germination without GA; scarification alone did not induce germination in this experiment (after seeds remained in the refrigerator another month, T. M. was able to get a few seeds to germinate with scarification alone and no GA, but the percent germination was low). Take-home: use both scarification and GA 300 ppm for this species. We used recently collected seeds, collected in May of 2015, for experiments in July 2015; seeds stored for a longer period may not require treatment. In another experiment Rebecca Flinn and T. M. were able to obtain many seedlings by both scarifying and treating J. calliantha seeds with 300 ppm for 20 to 21 hours. After soaking the seeds in 300 ppm GA, seeds were planted on the surface of potting mix, and kept moist and warm, with 24 hour lighting. After 10 days 7 out of about 22 seeds and 9 out of about 22 seeds germinated! |
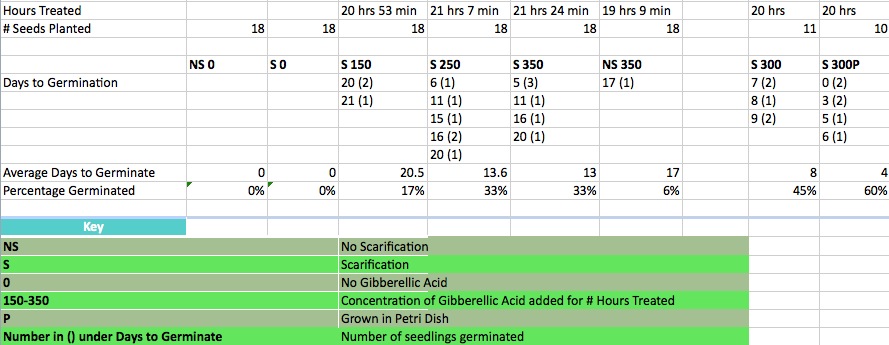
Seed germination in only 7 days: Seeds that were collected in May of 2015 (accession 855) were stored in a refrigerator until 20 Sept 2018 when seed coats were partially removed (while looking through a dissecting microscope), seeds were then treated with 300 ppm GA overnight, and then placed within a few mm of the surface of potting mix, and a plug-in warm mat was placed under the cups that were holding the seeds. First germinatation (one seedling) was recorded on 28 September 2018.
Floral Phenology Notes
| Data for one flower (tag 1) | phase | observations |
| Day 1, 4 Aug 2008 | pistillate |
Anthers undehsiced and cor open. No nectar in the morning, orange nectar in afternoon. Flower color very similar to leaves (unaided eye). Cor was closed yesterday. |
| Day 2, 5 Aug | hermaphroditic |
8 am - anthers have already dehisced, cor is fully open, orange nectar evident; 12:30 pm - stamens angle out orienting dehisced anthers away from stigma; 6 pm - same |
| Day 3, 6 Aug | hermaphroditic |
9 am - same as yesterday; noon - wide open; 7 pm - same |
| Day 4, 7 Aug | hermaphroditic |
8 am - cor wide open, lots of nectar; noon - cor only half open, seems to be closing at the end of its life |
| Day 5, 8 Aug | corolla closed, possibly self-pollinating |
9 am - cor changed color to brownish-green/greenish-brown. Cor closed in a manner that looks like it may bring dehisced anthers into contact with the stigma (speculation); noon - same; 11:30 pm - cor closed with parallel sides, color as at 9 am. |
| Day 6, 9 Aug | corolla closed |
8 am - cor brown and closed, cor not falling off when touched, closed in a manner having parallel sides; |
| Day 7, 10 Aug | corolla closed | noon - same; 6 pm - cor closed; 8 pm - cor shriveled but remaining on |
| Day 8, 11 Aug | corolla withering | 9 am - cor withering and brown, still present |
| Corolla was open for four days. Pollinators were excluded (by placing plant in car). Pistillate phase (phase 1) during its first day. Hermaphroditic phase (phase 2) during subsequent three days. Style quite rigid, possibly an adaptation to hummingbird visitation. |
Figure 38. Here is a paragraph about the importance of fieldwork. The specimen M. Weigend 2000/605 (shown above) collected in department Ancash was, when I first studied it, fascinating because flowers have a corona. At that time there were no Jaltomata species known from Department Ancash having a floral corona. I thought the specimen might be J. aspera or J. calliantha or J. quipuscoae because flowers of only these three species have a corona. It was a puzzle at the time, because J. quipuscoae was known only from Department Arequipa, J. aspera was known only from Department Lima, and J. calliantha was known only from Department La Libertad. Before me was a specimen, M. Weigend 2000/605, collected roughly half way between J. aspera and J. calliantha, geographically. The first approach I took to solving the mystery was to rehydrate a flower from the museum specimen M. Weigend 2000/605 and see if the rehydrated flower matched up morphologically with one of the species that has a floral corona. This approach did not provide a definitive answer. The rehydrated flower (above) seemed to lack 5 pairs of dark green corolla spots, lacking in J. aspera but present in both J. quipuscoae and J. calliantha. Had I stopped here I would have had the wrong answer! The second approach to solving the puzzle was to go to the place where Weigend 2000/605 was collected (photos labeled 855 and / or 856 above show the collection). Only with living plants, grown from seeds collected where Weigend collected, was I able to definitively and easily identify the species as J. calliantha. This new understanding led to an immediate (online) revision of the known geographic distribution of J. calliantha, with our new understanding being that J. calliantha grows both in department La Libertad and in department Ancash. Thus, a major revision in our understanding of the geographic distribution came about because Weigend discovered a new population and took the time to make a specimen and deposit it at an herbarium. Fieldwork by the discoverer of the population was not sufficient; fieldwork by both parties led to the understanding we have today. In other words, after Segundo Leiva G., Leon Yacher and I went to where specimen Weigend 2000/605 was collected, gathered seeds, and grew plants, I identified the living plants as J. calliantha with 100% certainty. Without fieldwork, a correct understanding of the geographic distribution of species would not have been possible. In the photo above, units are mm. A pressed/dry specimen was rehydrated in dilute detergent, and then removed from liquid for this photo by T. M. |